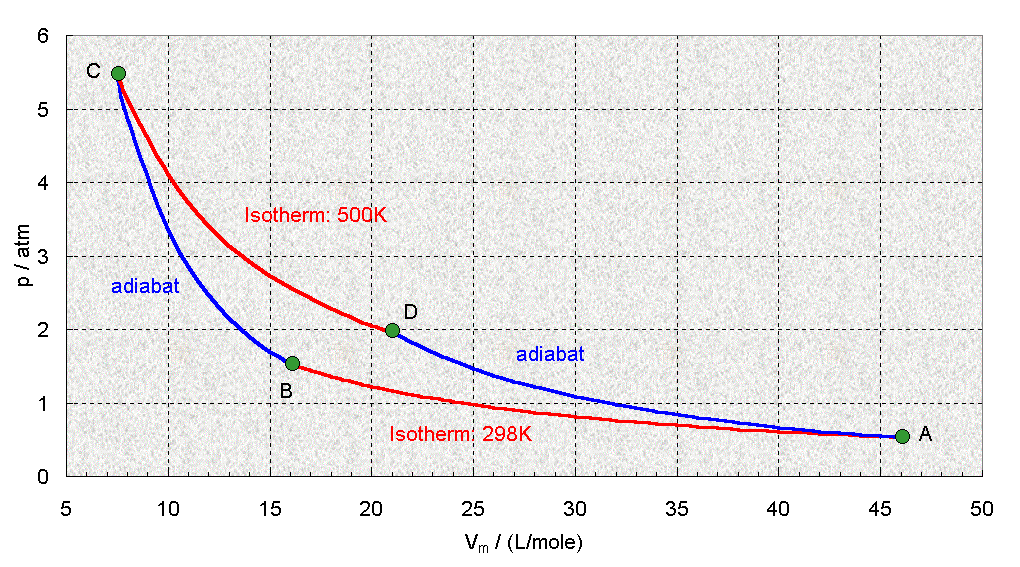
Carnot Cycle
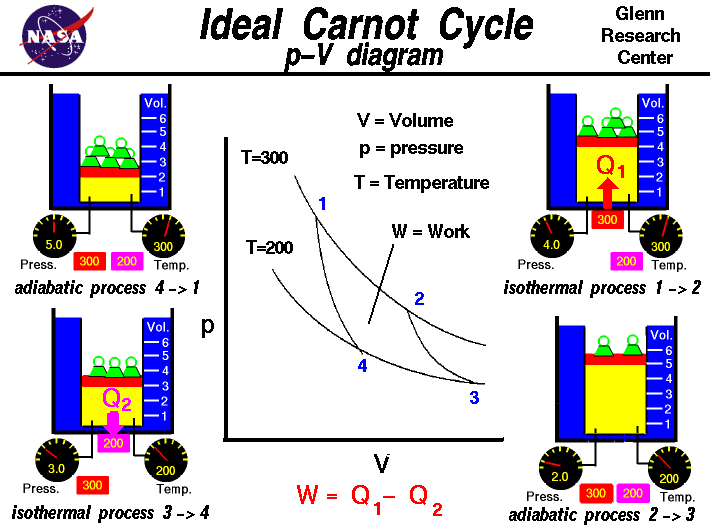
http://images.google.com/imgres?imgurl=http://www.grc.nasa.gov/WWW/K-12/airplane/Images/carnot.gif&imgrefurl=http://www.grc.nasa.gov/WWW/K-12/airplane/carnot.html&h=531&w=710&sz=26&tbnid=TZGMxU7dX0LTyM:&tbnh=105&tbnw=140&prev=/images%3Fq%3Dcarnot%2Bcycle&start=3&sa=X&oi=images&ct=image&cd=3 http://classweb.gmu.edu/sdavis/chem331/carnot.gif
Carnot Cycle
With the Carnot Cycle, Carnot was able to show that a heat engine which operated at an ideal, reversible cycle, that was between two energy reservoirs was the most efficient engine possible. Although this is physically not a real engine that can be built, it's ideally the most efficient engine there is and no engine can ever be made more efficent.
1 - 2) This is an isothermal process (constant temperature) in which the gas expands while it is in contact with the hot reservoir
2 - 3) This is an adiabatic process (no energy) in which the gas expands
3 - 4) This is an isothermal process in which the gas is compressed while it is in contact with the cold reservoir
4 - 1) This is an adiabatic process in which the gas is compressed